The decision to implement a sustained release or extended release formulation relies on the character in the situation getting treated and the desired outcomes. Right here’s why you may pick one over the other:
By knowledge the differences concerning SR and ER remedies, you can also make much more informed decisions regarding your healthcare and transform your General treatment working experience.
Niosomes are nanosized vesicles made up of nonionic surfactants and cholesterol that form when these compounds are dispersed within an aqueous medium. These lipid-based constructions are just like liposomes but vary in their composition, as niosomes use nonionic surfactants in place of phospholipids. The exclusive characteristic of niosomes lies within their capacity to encapsulate the two hydrophilic and hydrophobic drugs in their bilayer membrane.
Our staff has around 20 years of drug encapsulation working experience and will do the job intently along with you in developing long-acting injectables for your needs. Take a look at our homepage to understand every thing we can offer.
This document offers an summary of the seminar on sustained release drug delivery systems. It discusses: one. The introduction and strategy of sustained release drug delivery, including some great benefits of retaining a continuing drug level after some time. 2. The differences amongst controlled release and sustained release, with controlled release supplying exact control of drug release and sustained release prolonging drug stages for an extended time.
This get more info doc offers an outline of controlled release drug delivery systems (CRDDS). It defines CRDDS as systems that provide some control more than the temporal or spatial release of drugs.
Ways to structure-controlled release formulations determined by diffusion, dissolution and ion exchange concepts. Physicochemical and Organic Qualities of drugs applicable to controlled release formulations.
This document discusses several oral drug delivery mechanisms which includes dissolution controlled release systems, diffusion controlled release systems, and mixtures of dissolution and diffusion. It describes matrix and encapsulation dissolution controlled release systems check here along with matrix and reservoir diffusion controlled release systems.
This characteristic will help in Arranging and monitoring advanced initiatives by dividing operate into lesser ways, each with its own deadlines, assignees, and development monitoring.
Dosing Frequency: Due to the for a longer period release time, ER prescription drugs normally involve fewer doses—often just after each day—while SR medicines may must be taken two or maybe more times per day.
Floating systems consist of non-effervescent and effervescent forms that float resulting from low density or gas technology. Substantial-density systems do not float but remain while in the belly as a result of bioadhesion, magnetic forces, swelling to a substantial size, or raft development on gastric fluids.
Some samples of prescription drugs that are available within the prolonged-release tablet form consist of anti-hypertensive drugs like Metformin hydrochloride prolonged-release tablets.
This doc discusses aspects impacting the design of controlled release drug delivery systems (CRDDS). It outlines various important things to consider for CRDDS style and design which include variety of the drug applicant, medical and Organic rationale, and physicochemical Homes.
Spreadability: Distribute 1g of cream amongst two glass slides and measure the unfold diameter less than a specified load.
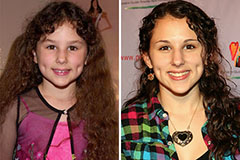 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Judd Nelson Then & Now!
Judd Nelson Then & Now! Judge Reinhold Then & Now!
Judge Reinhold Then & Now! Matilda Ledger Then & Now!
Matilda Ledger Then & Now!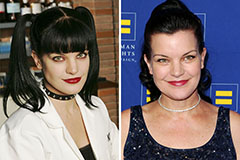 Pauley Perrette Then & Now!
Pauley Perrette Then & Now!